Currently licensed influenza vaccines offer very low levels of protection, despite annual review and updating of the vaccine composition.
In adults over 65 years there has been little to no detectable protection despite annual revaccination. The vaccines induce antibodies to highly variable regions of the major surface antigen haemagglutinin (HA), which can only contribute to protection if the virus that then causes an infection differs little from the vaccine components.
A far better solution would be to routinely use a broadly protective vaccine which is not susceptible to antigenic drift and can also protect against pandemic viruses. This may also reduce the need to vaccinate annually.
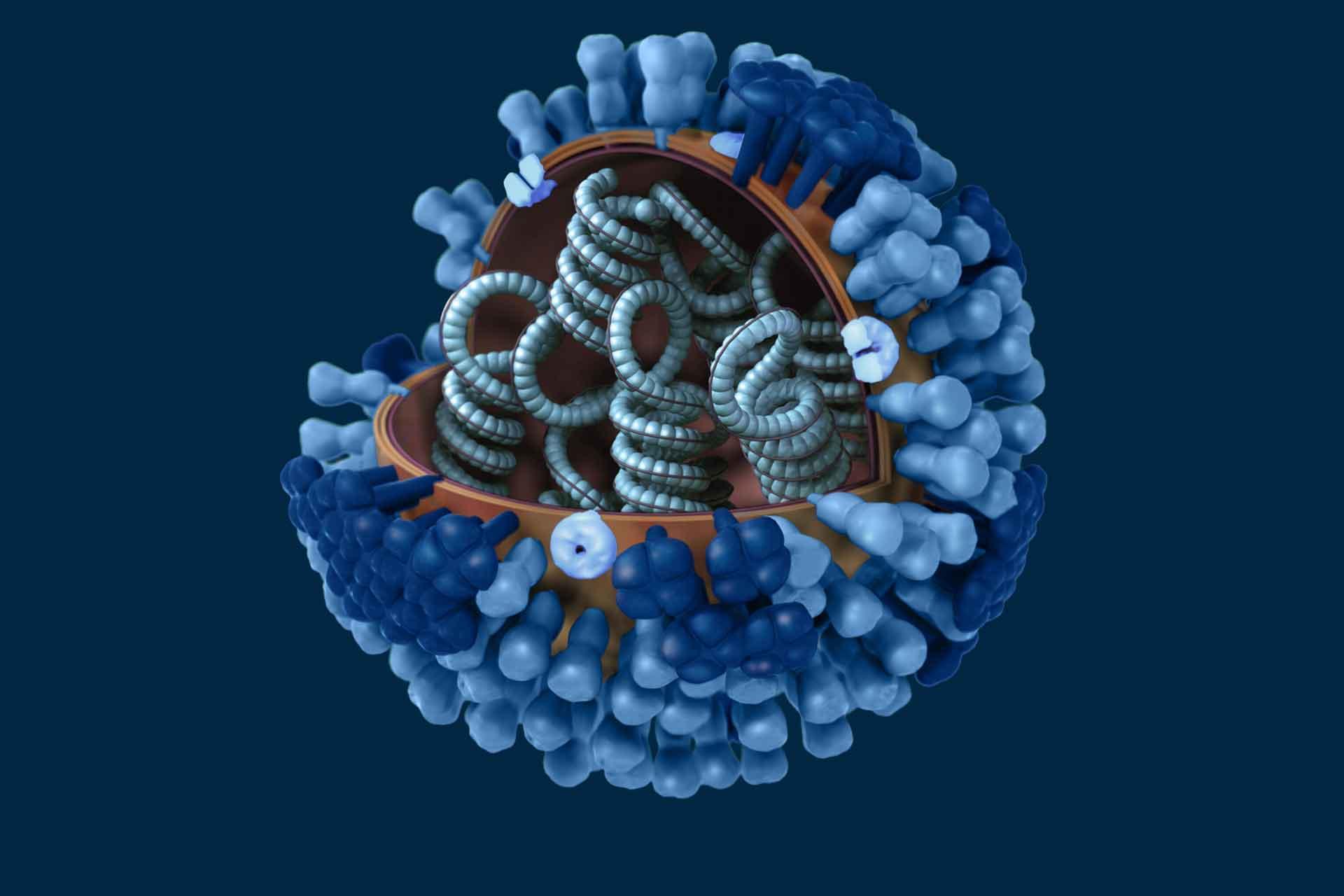
In contrast to the highly variable HA, the internal antigens of influenza are highly conserved between all influenza A viruses and inducing strong immune memory responses against these antigens would provide lasting protection against at least severe disease with both seasonal and potentially pandemic viruses. The proteins that are considered as suitable vaccine antigens are nucleoprotein (NP) and matrix protein 1 (M1), which are both expressed at high level after infection. Recently the neuraminidase (NA) has been the subject of much interest as a vaccine antigen since it is less variable than HA and antibodies to NA correlate with protection in animal models.
We have shown that priming flu-exposed pigs with ChAdOx2 expressing NP, M1 and NA, and boosted with MVA expressing the same three antigens, delivered by the intramuscular, intranasal or aerosol (lung) routes. However, the contribution of NP/M1 to vaccine efficacy compared to NA is unclear, nor is the optimal route of vaccine delivery.
In this programme of work answer these questions using the robust pre-exposure pig influenza model. This will inform vaccine design to produce a vaccine that could potentially replace current seasonal vaccines at least in low-income countries.
Collaborators
- Professor Sarah Gilbert, University of Oxford
- Professor Nicola Lewis, The Francis Crick Institute