Species determination of Culicoides biting midges via peptide profiling using matrix-assisted laser desorption ionization mass spectrometry
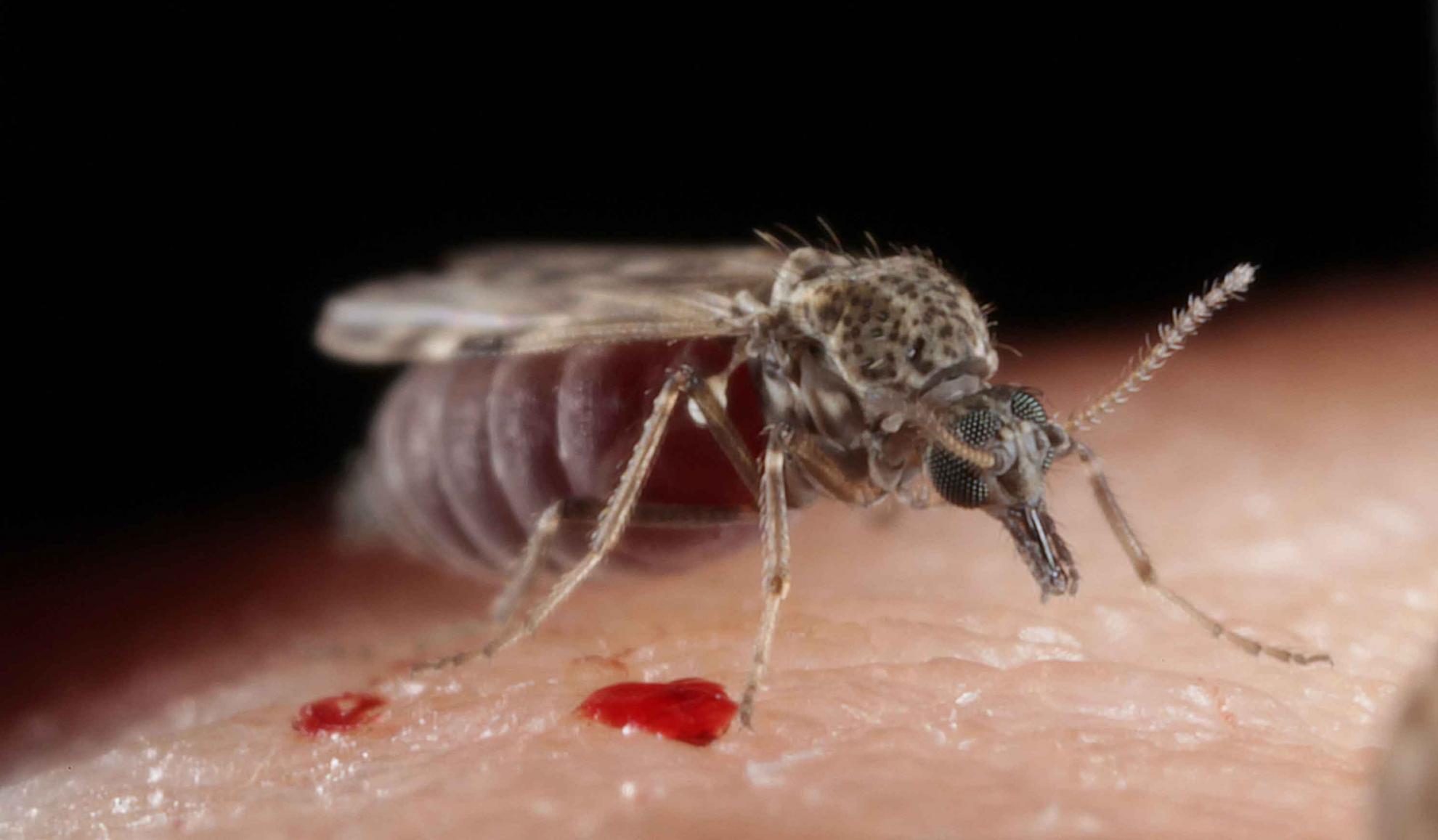
Background: Culicoides biting midges are vectors of bluetongue and Schmallenberg viruses that inflict large-scale disease epidemics in ruminant livestock in Europe. Methods based on morphological characteristics and sequencing of genetic markers are most commonly employed to differentiate Culicoides to species level. Proteomic methods, however, are also increasingly being used as an alternative method of identification. These techniques have the potential to be rapid and may also offer advantages over DNA-based techniques. The aim of this proof-of-principle study was to develop a simple MALDI-MS based method to differentiate Culicoides from different species by peptide patterns with the additional option of identifying discriminating peptides. Methods: Proteins extracted from 7 Culicoides species were digested and resulting peptides purified. Peptide mass fingerprint (PMF) spectra were recorded using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) and peak patterns analysed in R using the MALDIquant R package. Additionally, offline liquid chromatography (LC) MALDI-TOF tandem mass spectrometry (MS/MS) was applied to determine the identity of peptide peaks in one exemplary MALDI spectrum obtained using an unfractionated extract. Results: We showed that the majority of Culicoides species yielded reproducible mass spectra with peak patterns that were suitable for classification. The dendrogram obtained by MS showed tentative similarities to a dendrogram generated from cytochrome oxidase I (COX1) sequences. Using offline LC-MALDI-TOF-MS/MS we determined the identity of 28 peptide peaks observed in one MALDI spectrum in a mass range from 1.1 to 3.1 kDa. All identified peptides were identical to other dipteran species and derived from one of five highly abundant proteins due to an absence of available Culicoides data. Conclusion: Shotgun mass mapping by MALDI-TOF-MS has been shown to be compatible with morphological and genetic identification of specimens. Furthermore, the method performs at least as well as an alternative approach based on MS spectra of intact proteins, thus establishing the procedure as a method in its own right, with the additional option of concurrently using the same samples in other MS-based applications for protein identifications. The future availability of genomic information for different Culicoides species may enable a more stringent peptide detection based on Culicoides-specific sequence information.
Back to publications