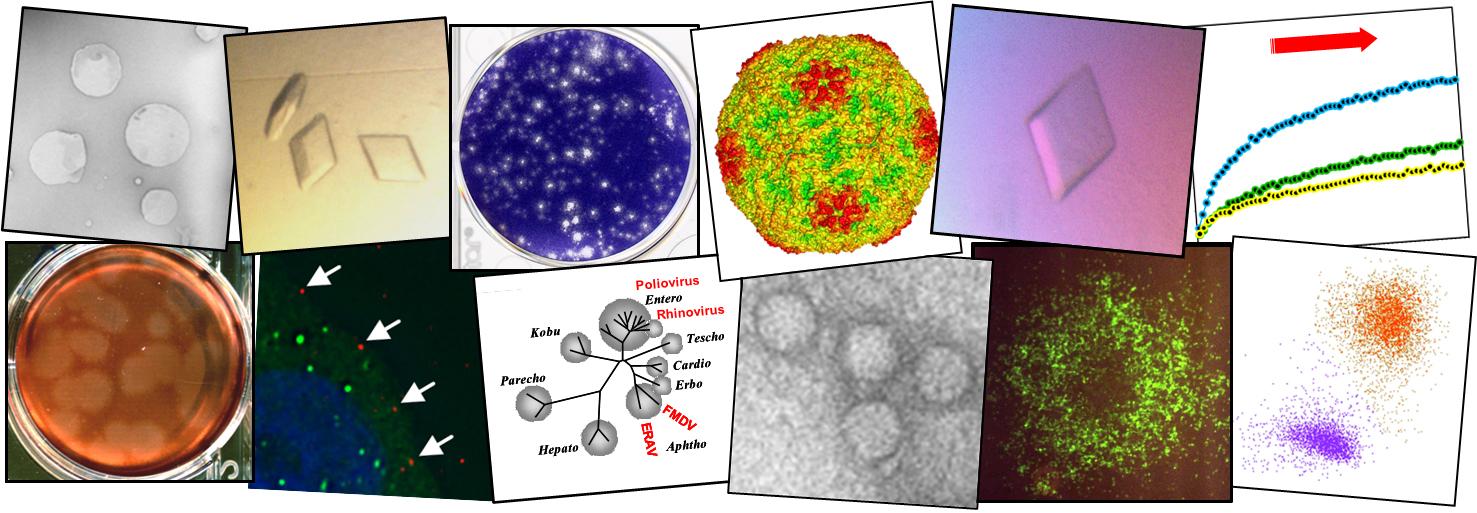
Capsid protein VP4 of human rhinovirus induces membrane permeability by the formation of a size-selective multimeric pore
Human rhinovirus (HRV) is a non-enveloped virus of the picornavirus family and is responsible for respiratory infections (common colds) costing billions of dollars ($) annually. There remains no vaccine or licensed drug to prevent or reduce infection. Related members of the picornavirus family include significant pathogens such as poliovirus, enterovirus 71 and foot-and-mouth disease virus, for which improved control measures are also required. A fundamental step in virus infection is the delivery of the viral genetic material through the barrier of the cellular membrane. Viruses such as HIV and influenza are enveloped in an outer membrane which can fuse with the host cell membrane to allow the viral genome to penetrate into the cytoplasm. However, non-enveloped viruses such as picornaviruses lack a membrane and the mechanisms for penetration of the membrane by these viruses remain poorly understood. The capsid protein VP4 has previously been implicated in the delivery of the picornavirus genome. In this study we demonstrate that HRV VP4 interacts with membranes to make them permeable by the formation of multimeric, size-selective membrane pores with properties consistent with the transport of viral genome through the membrane. This function of VP4 provides a novel antiviral target for this family of viruses.
Back to publications