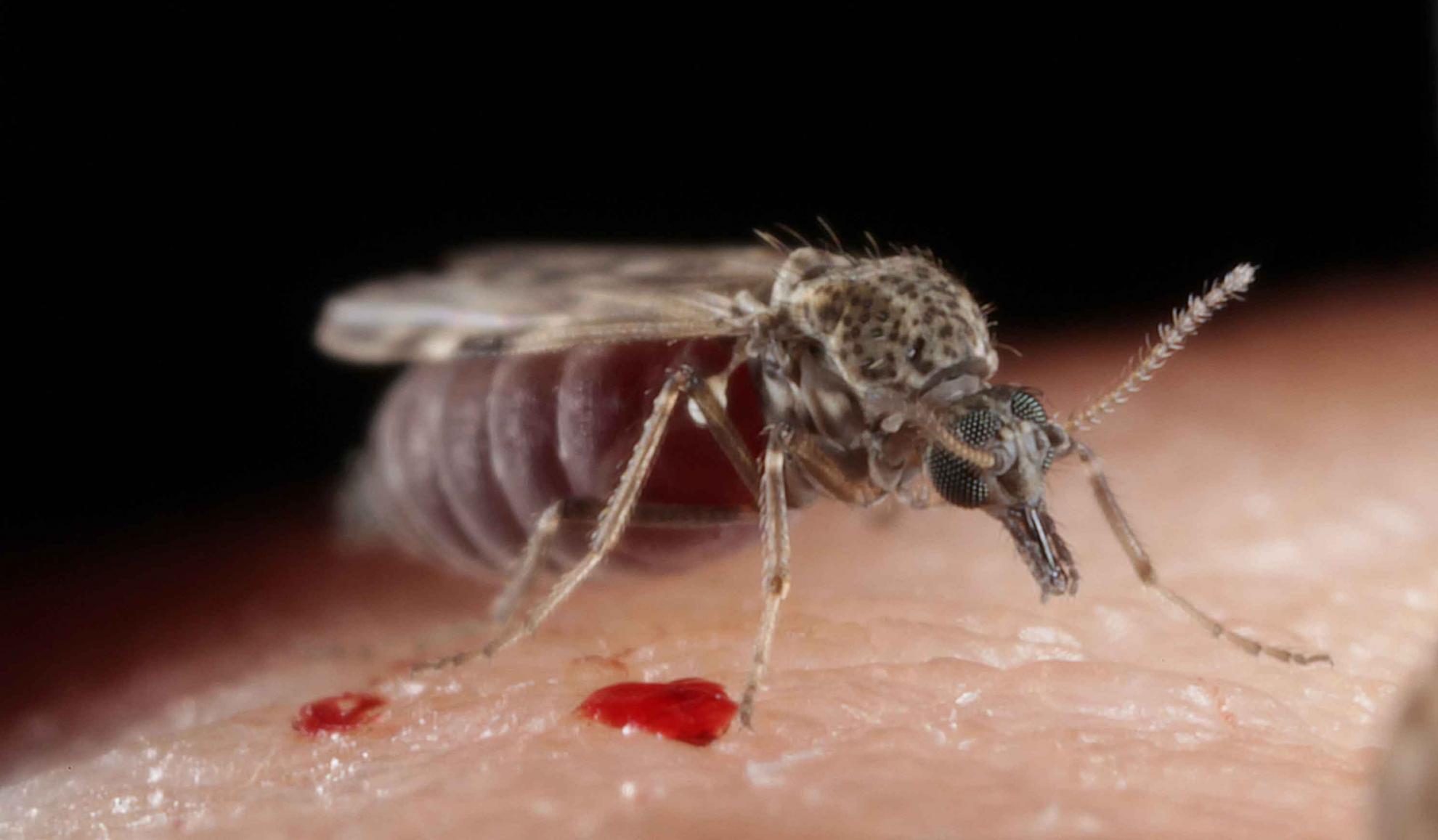
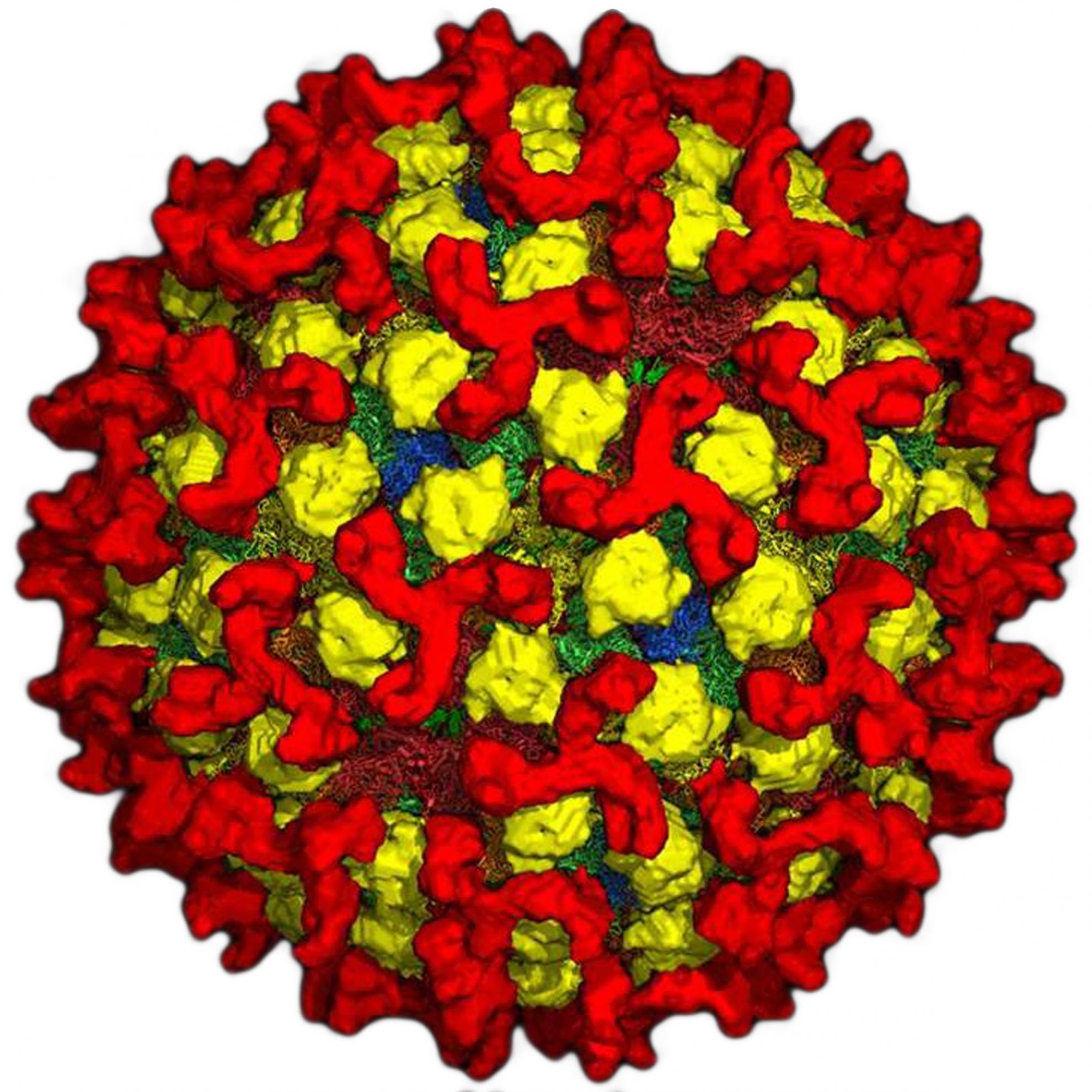
Bluetongue virus (BTV) is responsible for causing the severe haemorrhagic disease, bluetongue (BT).
It can infect domestic ruminants including cattle, sheep and goats, along with wild animals such as buffalo, deer, antelope and camels.
BTV is present in all continents except Antarctica. There have been two introductions of BTV into the UK, one in 2007 and one in 2023.
BTV is predominantly spread between ruminants through the bites of infected Culicoides midges, tiny blood-feeding insects that can be found in large numbers on most farms. Some BTV strains can be transferred from a ruminant mother to her foetus during pregnancy and a small number through direct contact between ruminants.
BT can result in high rates of morbidity and even mortality in flocks and herds, and can affect production (e.g. milk yields) and trade.
It has a high global economic cost and is controlled primarily through vaccination and movement restrictions.
For the latest information on the bluetongue situation in the UK and how to spot and report, please visit gov.uk.
For information on diagnostic testing, including pricing and submission forms, please visit our webpage. Please note: we are unable to advise on licencing requirements.
Clinical signs
Some breeds of sheep are particularly susceptible to BTV infection and show severe clinical signs of BT. Disease severity varies with the strain of BTV, but also by the individual animal, breed and species. Cattle and deer are less likely to show clinical signs but are thought to be important ‘reservoirs’ of the virus.
Clinical signs include:
- Fever
- Reddening of the mucosal membranes, eyes, and/or region above the hoof
- Sores on the nose, gum and/or dental pads
- Discharge from the nose
- Swelling of the face, lips, nose and/or tongue
- Lameness
- Depression
BT can also lead to death and can cause abortion or deformities in lambs or calves.
The “blue tongue”, from which the disease gets is name, is not seen frequently. Animals may also develop breathing difficulties if the tongue swells.
Virology
BTV is one of at least 22 recognised species in the genus Orbivirus, belonging to the wider Sedoreoviridae family. It is a complex, non-enveloped virus which has a double stranded RNA genome consisting of ten segments of different sizes. These segments encode its seven structural proteins (VP1-7) and five non-structural proteins (NS1-5). The BTV particle has an icosahedral structure, which consists of three layers including the outer capsid, core and sub-core.
Due to its segmented genome, BTV can reassort, swapping genome segments, when two BTV strains replicate within the same cell. This can give rise to new BTV strains, which can have different characteristics, and potentially virulence, which may make current vaccines ineffective.
There are at least 29 recognised serotypes of BTV, with strain diversity observed within each serotype. BTV serotypes 25-29 are considered ‘atypical’ as they are not spread by Culicoides biting midges, but can spread via contact, typically they do not cause clinical disease.
Vaccines are available in Europe for some BTV serotypes. However, these only protect against the BTV serotype targeted by the vaccine (i.e., not against all 29 serotypes).
Pirbright's research on bluetongue virus
Predicting
Together with Defra and the Met Office, we collate data on the disease situation in near-continental Europe, wind patterns and temperatures to assess the risk of wind-borne incursion of BTV-infected vectors into the UK.
We have developed mathematical models that allow us to understand and predict the transmission of BTV within and between farms. We use these models to advise Defra on the effectiveness of possible measures, such as movement restrictions and vaccination, to control the spread of BT disease.
Detecting
As home to the National and World Organisation of Animal Health (WOAH) reference laboratory for BTV, we provide an essential diagnostic, surveillance, and advisory service to the UK government and WOAH.
We are responsible for maintaining and continually improving the suite of molecular and serological tests that detect BTV in infected ruminants.
We are currently developing new diagnostic tests for BTV, including serological tests to predict time since infection and/or vaccination in ruminants.
Using the latest next generation sequencing technologies, we are improving the speed and efficiency of determining the genetic sequence of BTV isolates, which will help us to understand how viruses evolve, spread and the origin of new incursions or outbreaks.
Understanding
Our work examines the molecular biology of BTV to try to understand the influence of different BTV particle types on cell tropism and host and vector range. We also study the impact of viral co-infections on the evolution and diversity of BTV.
Using our high-containment animal facilities, we investigate the clinical presentation, pathology, infection dynamics and immune response in cattle and sheep infected with new and emerging BTV strains.
Our unique virus-vector-host transmission model allows us to investigate the efficiency of transmission to and from Culicoides midges to predict likely spread of BTV.
We are also working to characterise the ruminant immune response to BTV infection and vaccination, with a specific focus on T cells, B cells and antibody dynamics.
We investigate how Culicoides biting midges transmit BTV between animals. Using our Culicoides midge colonies, we can explore many aspects of BTV transmission. For example, we have developed methods to see where BTV replicates inside midges to find out why some midges can transmit BTV when others cannot.
We also monitor the activity of adult midges across the UK at key times in the year to understand when disease transmission may be possible.
Responding
We help Defra respond to BTV outbreaks through:
- Screening ruminant blood samples with our suite of molecular and/or serological tests to detect BTV-infected animals, identify the responsible BTV serotype and its potential origin.
- Monitoring vector activity and temperatures to assess BTV transmission risk at different times of the year.
- Using mathematical models to predict the spread of BTV and the potential impact of specific control measures.
- Providing expert advice on the disease, the virus, and its vectors.