Scientists at The Pirbright Institute have shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease (COVID-19), is able to enter cells using the receptors of multiple mammalian species. The broad host range of SARS-CoV-2 confirms the potential risk of infection to a wide range of companion animals, livestock and wildlife.
Like other human coronavirus diseases such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), COVID-19 is a zoonotic disease that has jumped from animals into humans. Identifying the original animal reservoir for SARS-CoV-2 and intermediate hosts which spread the virus to humans may ultimately help to understand how, where and when this virus spilled over into humans. This knowledge could be vital in preventing subsequent outbreaks of both related and unrelated viruses.
To enter cells, SARS-CoV-2 uses its spike glycoprotein to bind to ACE2 receptors on the surface of cells in various tissues including the lungs, heart and gastrointestinal tract. Researchers experimentally infected cells displaying human ACE2 or a panel of related ACE2 receptors from 22 animal species (including birds, rodents, companion animals and livestock).
Their pre-published study demonstrates that the spike glycoprotein uses ACE2 receptors from each species differently, but that dog, cat and rabbit ACE2 receptors were utilised most effectively. Efficient entry could potentially correlate with infection being more easily established in these animals.
However, it is important to note that SARS-CoV-2 cell entry is only the first step in viral transmission between different animal species. An animal’s susceptibility to infection and its subsequent ability to infect others is reliant on a range of factors such as whether the SARS-CoV-2 is able to replicate once inside cells and the animal’s ability to fight off the virus.
Interestingly, although bats are suspected to be the original reservoir of SARS-CoV-2, the results show that receptors from three bat species were among those that were least efficiently used by the spike glycoprotein. This is possibly due to adaptations SARS-CoV-2 has made during its zoonotic emergence in order to effectively bind to human ACE2 receptors.
More thorough investigations and detailed experimental animal challenge studies are required to ascertain whether livestock and companion animals could be receptive to COVID-19 infection from humans and act as reservoirs for this disease.
Dr Dalan Bailey, Head of the Viral Glycoproteins group at Pirbright said: “Our research identifies species where viral entry is most efficient, allowing scientists to prioritise research on animals that might be susceptible to disease. We also identify animals that could provide good experimental models for understanding COVID-19, such as hamsters and rabbits.”
This research was funded by the Medical Research Council, the Biotechnology and Biological Sciences Research Council, Innovate UK Department for Health and Social Care, the Royal Society and the Wellcome Trust.
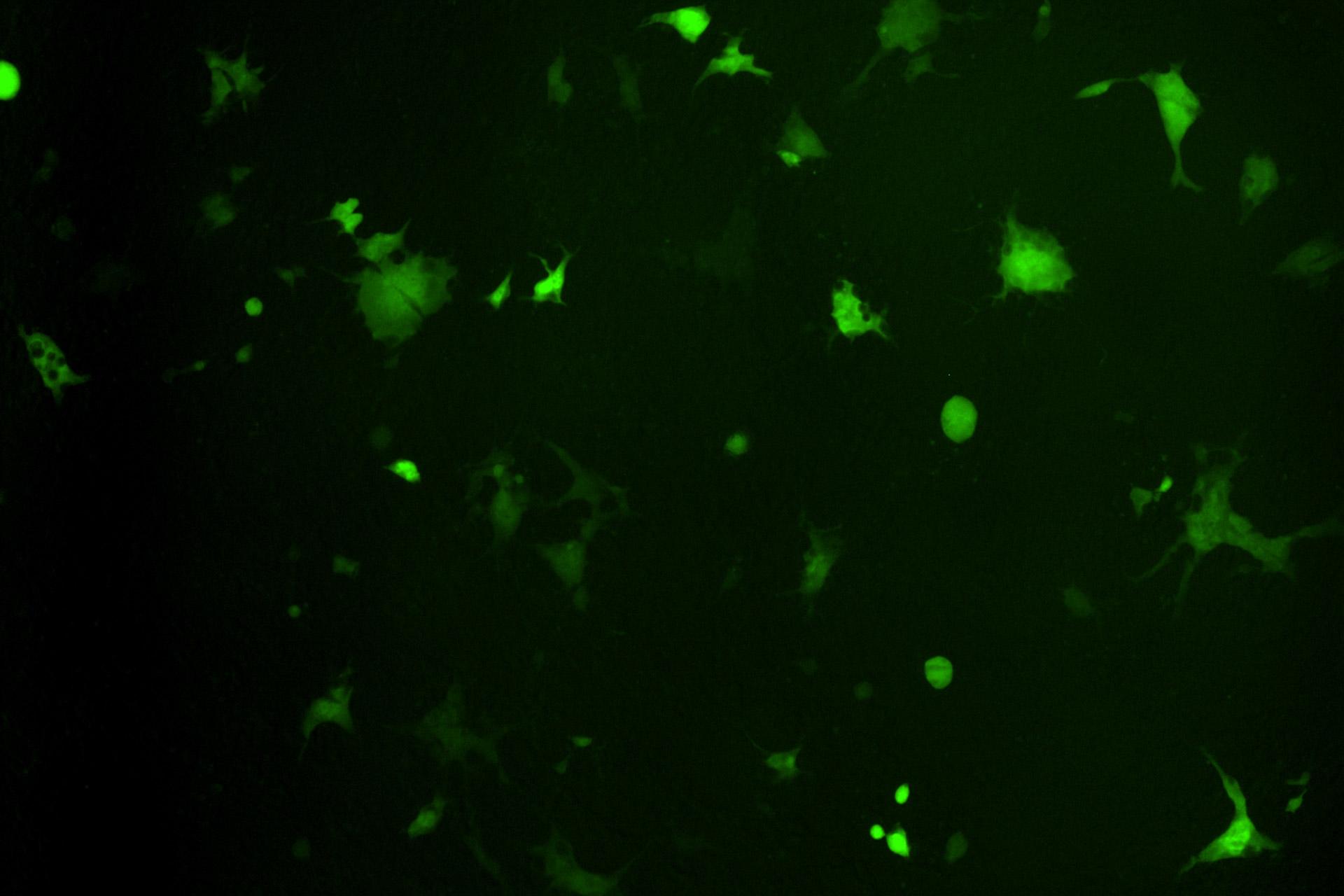
Image shows merged cells following co-expression of the SARS-CoV-2 spike glycoprotein and human ACE2 receptors. The spike glycoprotein induces cell-cell fusion after engaging with ACE2, allowing a fluorescent protein to become activated.