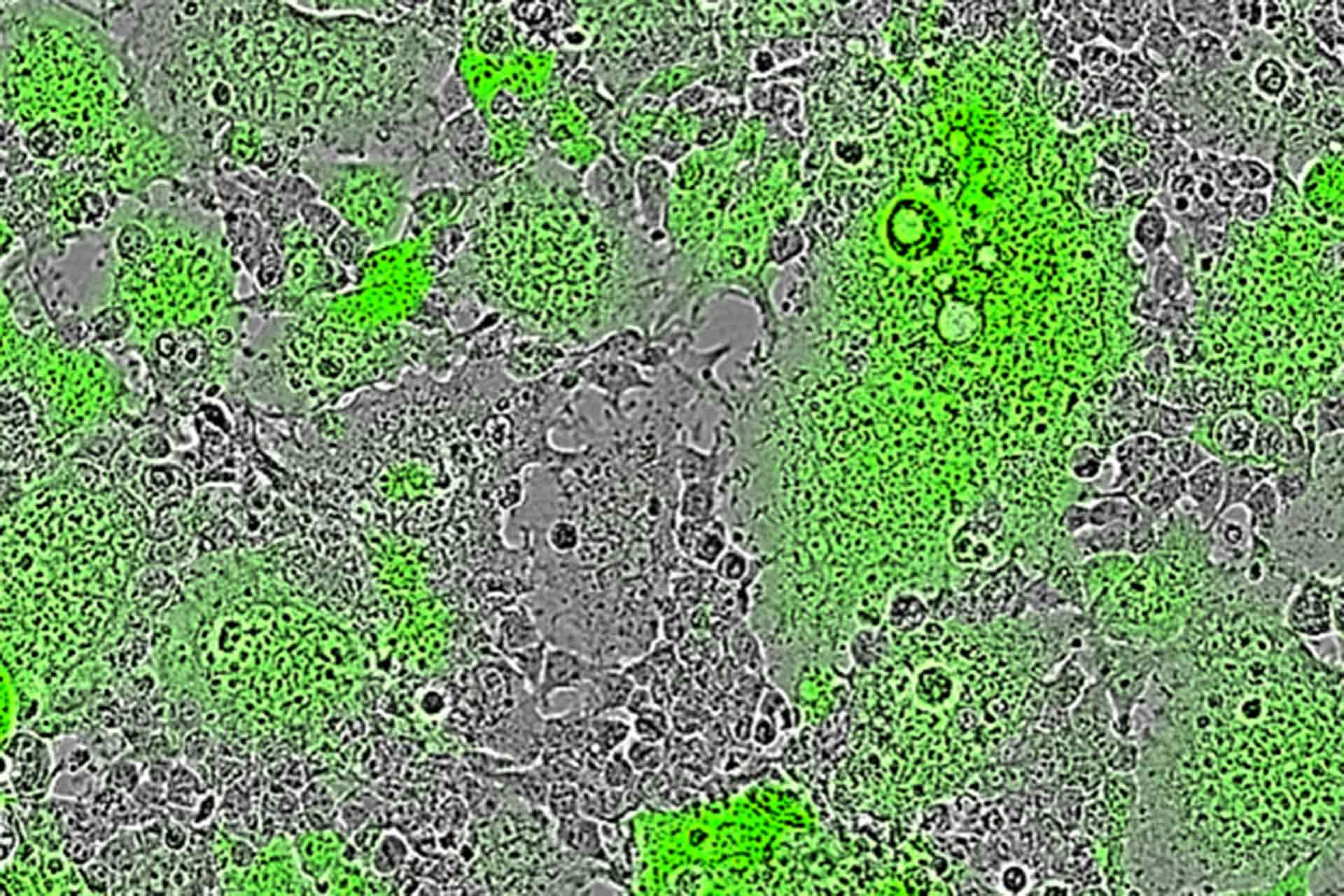
A new test developed by researchers at The Pirbright Institute, in collaboration with the University of Queensland and the University of Oxford, can detect antibodies that prevent cell-to-cell fusion, a method some viruses use to infect neighbouring cells. The new test is a valuable tool for analysing the antibody response against multiple viruses, including respiratory syncytial virus (RSV), Nipah virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19. The method can be used in tandem with other tests to assess whether vaccines and antivirals produce an effective immune response.
Most antibody tests focus on detecting neutralising antibodies, which block viruses from entering cells to prevent infection. However, some viruses have evolved a way to infect neighbouring cells without needing to form viral particles. Instead, their viral surface proteins recognise receptors on adjacent cells, forcing them to fuse together. This creates large cells with multiple nuclei, known as syncytia, and may allow the virus to spread in a way that avoids neutralising antibodies.
Cell fusion is used by viruses such as RSV and Nipah virus to spread, and there is growing evidence that SARS-CoV-2 can also cause syncytia formation. In their paper, the researchers demonstrated that the spike protein of SARS-CoV-2 can induce cell to cell fusion; however, the importance of this to natural infections remains to be determined. The immune response to these fusion events is poorly understood partly owing to the limited tools for investigating antibodies which may prevent syncytia formation.
The new method, named the micro-fusion inhibition test (mFIT), could be the very tool to fill this gap. Studies described in the Journal of General Virology demonstrate the test can be used in a variety of applications to assess whether antibodies can halt fusion events.
This included assessing therapeutic antibodies for RSV and Nipah, evaluating antibodies raised in response to Nipah vaccines in pigs and examining the ability of SARS-CoV-2 therapeutics to prevent cell fusion. The mFIT was also used to show that antibodies from recovered COVID-19 patients could prevent the formation of syncytia, allowing the team to characterise an important sub-class of SARS-CoV-2 antibodies, which may provide an additional indication of immunity.
Pirbright scientists are now actively using mFIT to assess a number of COVID-19 vaccines to provide more in-depth information about the immune responses triggered, as part of extensive collaborations within the vaccine development field.
Dr Dalan Bailey, head of the Viral Glycoproteins Group at Pirbright said: “The broad applications of this test, alongside its reliability and high throughput nature, make the mFIT a valuable tool that can be used in tandem with standard neutralisation tests to provide new insights into the importance of cell fusion in infection and immunity. This test gives us additional markers to assess when developing vaccines and therapies and could ultimately help us to improve their effectiveness.”
This research was funded by the Medical Research Council (MRC) and the Biotechnology and Biological Sciences Research Council (BBSRC), both part of UK Research and Innovation (UKRI). This research was also funded by the Department of Health and Social Care as part of the UK Vaccine Network (UKVN), a UK Aid programme to develop vaccines for diseases with epidemic potential in low and middle-income countries (LMICs).
Image shows Nipah virus induced cell-cell fusion leads to the formation of fluorescent green syncytia (multi-nucleated cells) in Pirbright’s mFIT assay.