Scientists from The Pirbright Institute have worked with UCL to map the expression of genes across the entire African swine fever virus (ASFV) genome, which has helped to establish their order of activation as well as uncovering new genes. The research could provide vital information for those developing vaccines and antiviral drugs to prevent the deadly pig disease caused by this virus.
ASFV causes an often lethal haemorrhagic fever in domestic pigs and wild boar. Major African swine fever outbreaks continue to spread across Eastern Europe, Asia and Africa, which in 2019 resulted in the death of nearly seven million pigs. In the absence of a vaccine or antivirals, the only way to prevent outbreaks is through biosecurity measures.
In their study, published in the Journal of Virology, the researchers used next generation sequencing to analyse genes expressed by ASFV. From this they created the first complete genetic road map, which reveals the order that different sets of ASFV genes are turned on throughout its infection cycle.
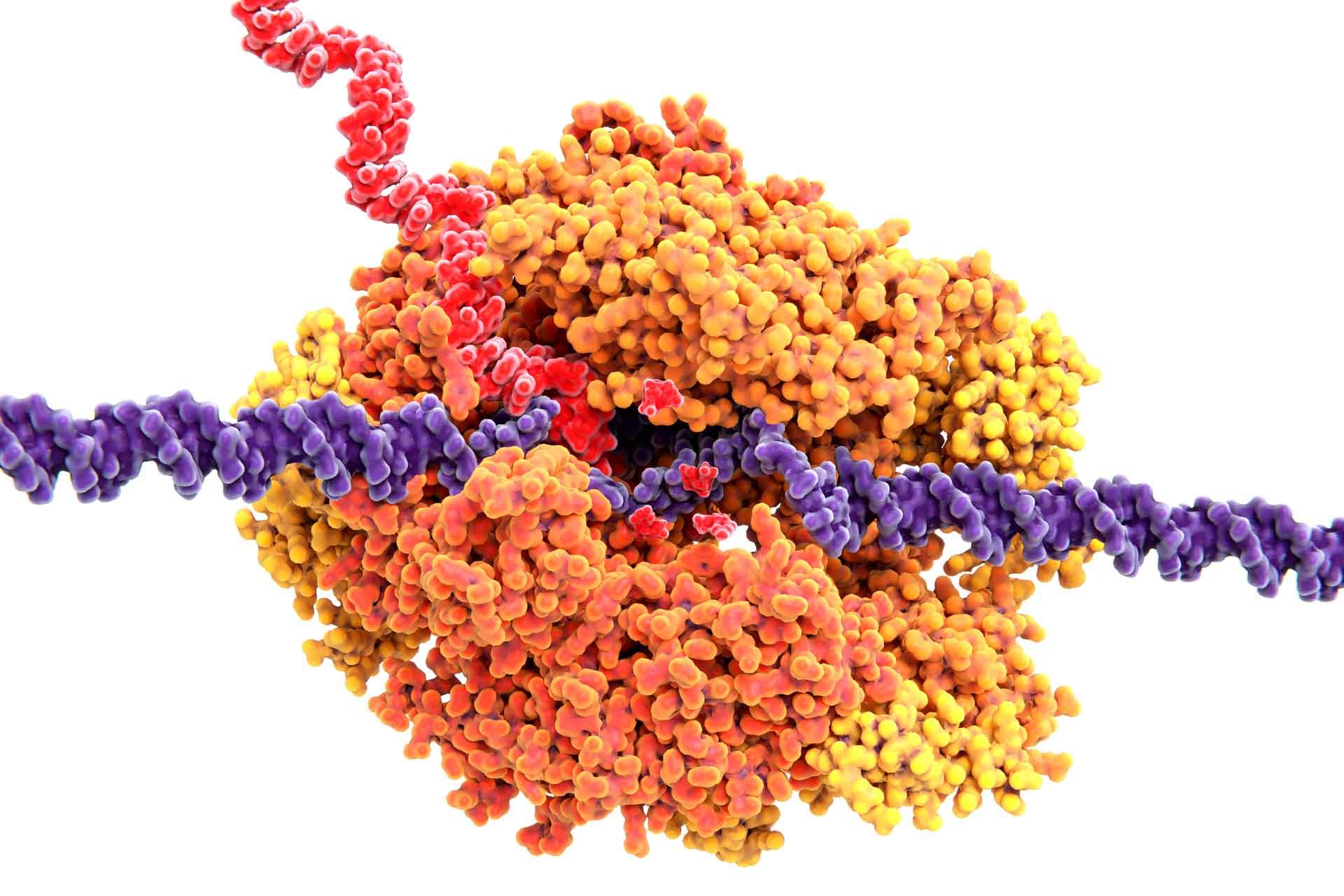
Genes, including those in ASFV, are activated through a process called transcription. This is carried out by a molecular machine called RNA polymerase, which serves as ‘gatekeeper’ by ensuring that the information coded in the DNA is expressed at the correct time during infection. The RNA polymerase finds genes based on specific DNA sequences, or ‘promoters’, that are located before a gene.
The team demonstrated that genes expressed during early infection have different promoters to those expressed later, allowing the virus to shift the pattern of activated genes according to the stage of infection. Genes used for DNA replication and immune system evasion are switched on early in the infection cycle, whereas those involved in creating proteins for the new virus particles are activated later.
“ASFV has a very large DNA genome. For comparison, the influenza virus expresses eight genes, whereas ASFV expresses between 150 and 190, which has so far made it difficult for scientists to identify and determine the significance of each gene. Our study helps to untangle which genes are important during different stages of infection to better understand their functions”, said Dr Linda Dixon, Head of the African Swine Fever Virus Group at Pirbright.
“Our data shows ASFV has a complex and mammalian-like method for controlling gene expression, that uses specific promoters to enable RNA polymerase to differentiate between which genes it should express when during viral infection. Our study has also uncovered over 30 novel genes that were previously unknown”, said Finn Werner, Professor of Molecular Biophysics at UCL.
By advancing knowledge of ASFV fundamental biology, this study provides vital information that will help to progress research into desperately needed disease control methods.
This research was supported by funding from Wellcome.
Find out more about how you can help prevent the spread of African swine fever and Pirbright’s vaccine and antiviral research at www.pirbright.ac.uk/protect-our-pigs.
Image: The process of gene activation through transcription. RNA polymerase (yellow/orange) unwinds DNA (violet) and uses the code to build an RNA strand (red). The RNA can then be used to create viral proteins that have many different functions.