A new study from scientists at The Pirbright Institute and collaborators at the University of Surrey provides evidence that infectious bronchitis virus (IBV) can regulate the signals involved in the stress response, which would otherwise prevent a virus from making new copies of itself.
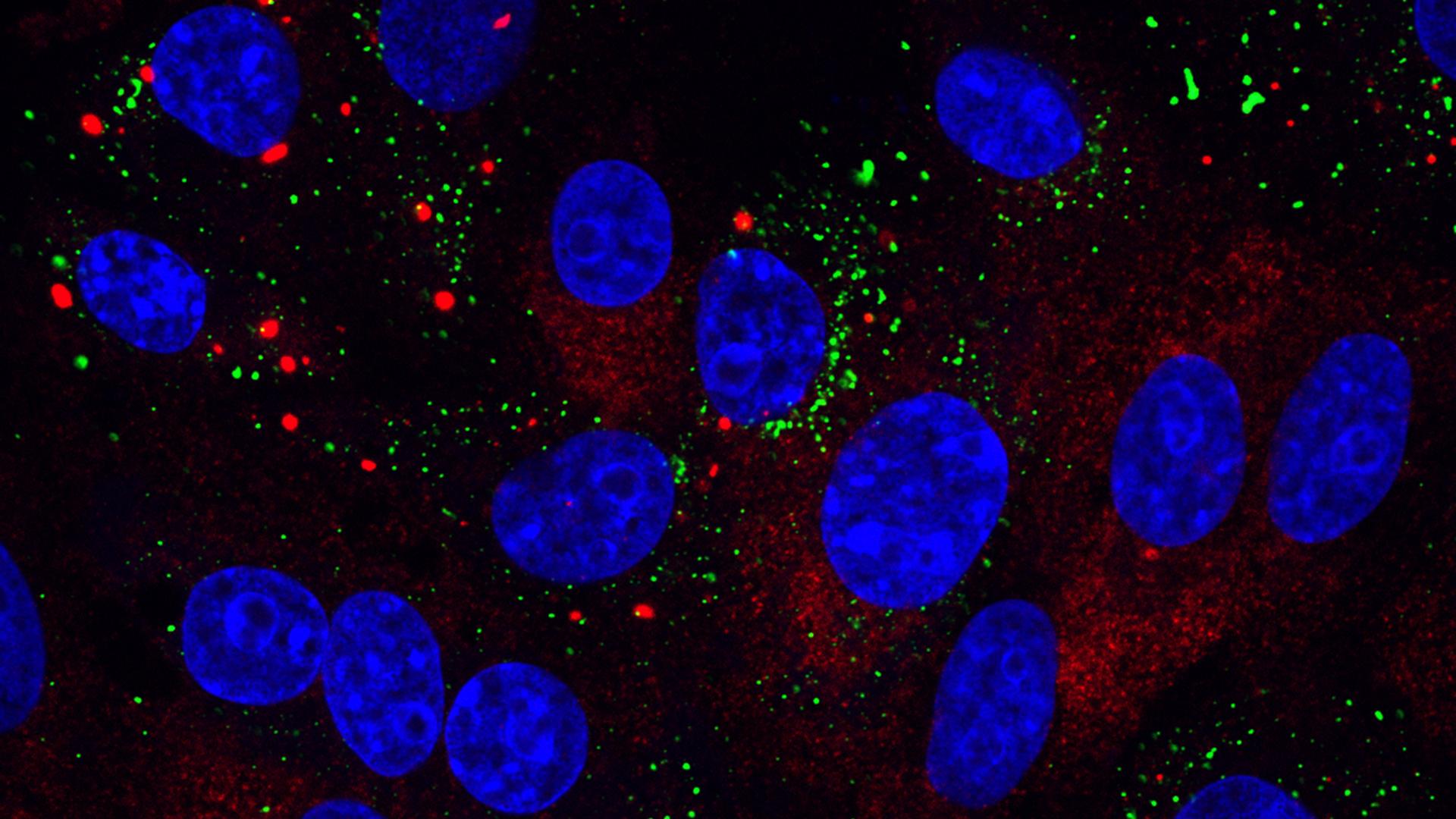
Their research, published in the journal Viruses, showed that stress granules, structures formed in the cell in response to stresses such as virus infection, were present only in a small proportion of infected cells. They also showed that IBV can actively inhibit stress granule formation.
IBV is a coronavirus that specifically infects birds, causing an economically important respiratory disease that leads to reduced egg production and meat quality in poultry. When IBV or other viruses enter a cell and begin to replicate, they hijack the cell’s own machinery to make copies of their genetic material and proteins. This includes hijacking the cell components involved in translation, the process by which new proteins are made.
In response to viral infection, cells can activate the stress response, which shuts down translation and stops the production of new proteins, except for those that are involved in the antiviral response. Cells then store the inactivated components of translation in stress granules.
In this new study a low number of IBV infected cells contained stress granules and IBV was shown to actively prevent their formation. The team therefore investigated the characteristics of these stress granules to understand why their suppression may be beneficial for the virus.
The stress granules do not overlap with sites of viral replication, suggesting that IBV is not hijacking these cellular components for its own benefit. As no viral RNA could be found in the stress granules it also seems they are not targeting the viral genome to prevent its replication, though the fact IBV has mechanisms to disrupt stress granule formation infers that they may be acting in another antiviral capacity.
In common with many other viruses, IBV replication itself results in the shut-off of translation at later stages during infection, which prevents the cell from making proteins involved in an antiviral response. This would normally go hand-in-hand with an increase in stress granule formation. Instead the researchers found that stress granule formation remained constant and the amount of IBV infected cells containing stress granules did not change.
These results show that IBV can uncouple several important cellular signalling pathways to its own advantage at different times during infection.
Dr Helena Maier, Head of the Nidovirus-Cell Interactions Group at Pirbright said: “IBV is doing something quite complex, it seems to uncouple and dysregulate lots of different signals in the cell, including those involved in the formation of stress granules. These are interesting observations and they have shown us that there is much more to tease out about the mechanisms IBV uses to regulate different signalling pathways.”
“Understanding the interactions between IBV and the host cell during infection provides information that is essential for future applied studies such as antiviral development. Knowing the mechanisms and pathways involved will allow researchers to use a more targeted approach, which could generate more effective treatments”, finished Dr Maier.
The study was funded by the Biotechnology and Biological Sciences Research Council, part of UK Research and Innovation (BBSRC UKRI).
Image: Infectious bronchitis virus (IBV) infection induces stress granules in a proportion of infected cells. Stress granules are stained using anti-G3BP1 antibody (red), IBV infection was detected using anti-dsRNA antibody (green) and nuclei were stained with DAPI (blue).