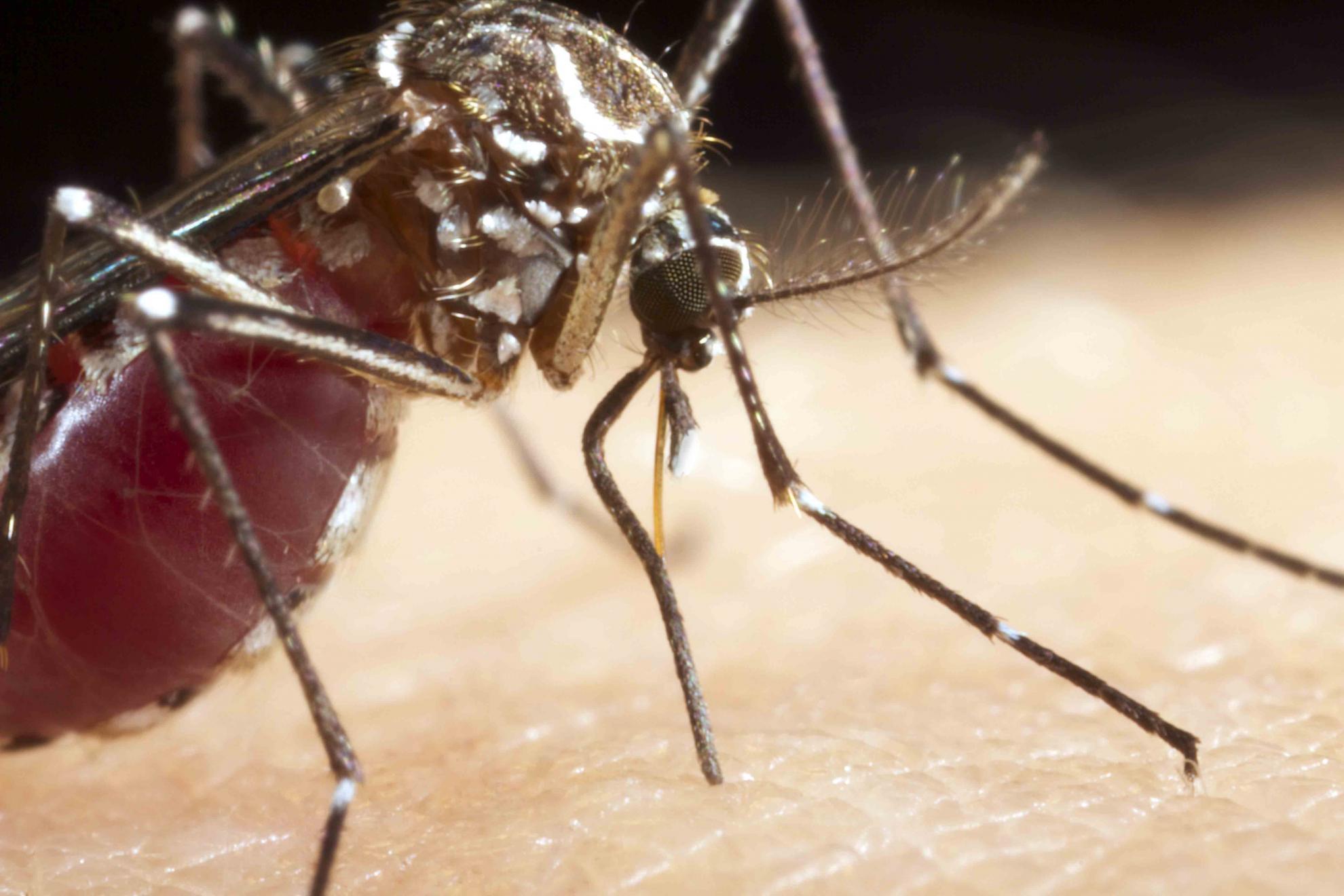
The Pirbright Institute has received an award worth up to $2.6 million to develop proof-of-concept tools that could prevent mosquitoes from transmitting a broad range of viruses. The project forms part of DARPA’s Preventing Emerging Pathogenic Threats (PREEMPT) programme, which aims to predict and contain viral mutations to prevent cross-species transmission of viral infectious disease from animals and insects to humans.
The research, led by Pirbright’s Professor Luke Alphey, will modify mosquitoes to reduce their ability to spread flaviviruses such as Zika virus, dengue virus, West Nile virus and yellow fever virus. This group of viruses has a wide range of hosts and can be spread from animals to humans (called zoonosis). “High profile emerging diseases such as Zika demonstrate how little we know about the hosts and vectors this group of zoonotic viruses usually occupy”, said Professor Alphey. “Predicting which viruses may next threaten humans is extremely difficult, and virus-specific therapies take time to develop, so we are usually on the back foot in terms of responding.”
The Pirbright team will be working with University of Nottingham and University of Tartu, Estonia, to counter this threat by developing entirely new methods of blocking viral replication in mosquitoes, thereby preventing the mosquitoes from transmitting viruses amongst host animals. “We aim to develop broad-spectrum tools that will inhibit transmission of both known and unknown viruses within this group, allowing rapid response or pre-emption of emerging threats” added Professor Alphey.
State-of-the-art genetic mechanisms will provide resistance to flaviviruses through the expression of anti-viral genes which will only be activated when a mosquito cell becomes infected. The team will also study scalable methods for propagating flavivirus resistance throughout mosquito populations.
Interrupting the transmission cycle of flaviviruses would provide a valuable method for reducing reservoirs of viruses that circulate in often hard-to-reach host animal populations, which in turn could lower the risk of spillover into humans. The broad spectrum action of the tools produced would allow multiple flaviviruses to be targeted at once.
“Reducing host animal reservoirs can be extremely difficult, particularly in areas where resources and technology for surveillance are hard to come by and the animal populations are remote. By targeting mosquitoes we can knock out the main path of transmission for flaviviruses and reduce the available pools of infection. This in turn would lower the chances of both existing and unknown flaviviruses spreading to humans”, added Professor Alphey.
The team’s approaches are now at an early stage of development. No field trials or releases are envisioned within any of these current projects, and all of the research will take place in biosecure facilities. The ultimate aim is to develop potentially field-usable tools for disease-control agencies in affected countries, and the data gathered could factor into decisions by those end users on what strategies to pursue to prevent new zoonoses.